PH/ORP Sensor Guide
Introduction

pH and ORP probes are both used for measuring the acidic intensity of liquid solutions. A pH probe measures acidity on a scale from 0 to 14, with 0 being the most acidic and 14 being the most basic. Similarly, an Oxidation-Reduction Potential (ORP) probe returns a voltage proportional to the tendency of the solution to gain or lose electrons from other substances (which is linked directly to the pH of a substance).
Note: You should avoid using multiple probes in the same solution, as they can electrically interfere with one another. If you are experiencing device instability or erroneous measurements, try removing other electrical devices (other sensors, electric pumps, etc) from the solution and try again.
How they work
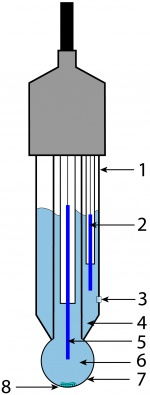
Both pH and ORP probes work by creating a small voltaic cell (battery) between the fluid they contain and the solution they are immersed in. The measured voltage is then converted into pH or oxidation/reduction potential.
Modern pH probes have the following components:
- The body of the electrode, typically made from non-conductive glass or impact resistant plastics.
- The reference electrode, usually comprised from the same metals as the main electrode.
- The junction zone between the studied solution and the reference solution, usually made from ceramics or a capillary with asbestos or quartz fiber.
- The reference solution, usually 0.1 mol/L KCl
- The internal electrode, usually made of silver chloride or calomel (Mercury(I) chloride).
- The internal reference solution, neutral buffered solution of 0.1 mol/L KCl.
- The sensing part of electrode, a bulb made from a special type of glass.
- When using a silver chloride electrode, a small amount of AgCl can precipitate inside the glass bulb.
Types of electrodes
The two types of probes are pH and ORP. They are constructed in more or less the same way, the only difference being the chemical they contain to form the battery with. The different chemical means they measure a different value. The only decision to make when choosing between a pH probe and an ORP probe is what scale you want the output to be in.
In general you can use any type of electrode with the ADP1000 - pH Phidget or the 1130 - pH/ORP Adapter which connects via a BNC connector, pH, ORP or otherwise. A conductivity electrode that uses a BNC connector may also work with this adapter as well. Just be sure that the output of the probe falls within the accepted input voltage range for the adapter.

Electrode Care
Electrode Calibration
Since glass pH electrodes measure H+ concentration relative to their reference half-cells, they must be calibrated periodically to ensure accurate, repeatable measurements. Although calibration against one pH reference buffer (one-point calibration) typically ensures accurate pH measurement, frequent two-point or even three-point calibrations ensure the most reliable results. Make sure your pH system includes calibration buffers for a range of pH values.
Conditioning
pH electrodes are shipped with the electrodes moist. Prior to using your electrode for the first time, follow these three steps to condition your electrode:
1. Remove the protective cap or rubber boot from the bottom of the sensor and rinse the electrode with distilled or deionized water.
2. Place the electrode in a beaker containing one of the liquids listed below (in order of ionic ability to condition the electrode). Soak for 20 minutes.
- 3.8 M or 4.0 M KCL
- 4.0 pH buffer
- 7.0 pH buffer
- Note: Never condition a pH electrode in distilled or deionized water. Long term exposure to pure water will damage the special glass membrane.
3. After conditioning the sensor for 20 minutes, rinse the electrode with distilled or deionized water. The electrode is now ready for calibration and to measure pH.
Handling
Electrodes should be rinsed between samples with distilled or deionized water. Never wipe an electrode—wiping can cause erroneous readings due to static charges. Blot the end of the electrode with lint-free paper to remove excess water. Never directly touch the glass membrane of the electrode with your fingers as oils from your hands will damage the electrode.
During shipment it is possible for air bubbles to move into the glass bulb. To remove air bubbles, shake down the electrode in the same manner as a clinical thermometer until the glass bulb is filled with solution.
Storage
Always keep your pH electrode moist. We recommend that you store your electrode in a storage solution. If storage solution is not available, use a pH 4 or 7 buffer solution. DO NOT store electrode in distilled or deionized water—this will cause ions to leach out of the glass bulb and render your electrode useless. After storage, you may notice white KCl crystals forming outside your electrode. This will not interfere with measurements. Simply rinse the electrode and blot dry before use.
If the pH electrode should dry out, soak the electrode up to 2 hours in pH buffer 4.0 solution. If the electrode is left dry for an extended period of time, you may need to soak it overnight before it will provide accurate readings.
Protective Plastic Bottle
Our electrodes are shipped with a protective plastic bottle over the glass bulb to help prevent cracking or scratching. Remove the plastic bottle before using your electrode. Keep your electrode in long-term storage with the bottle screwed on—just fill the bottle with enough storage solution to cover the glass bulb and replenish as needed to keep the bulb moist.
Cleaning
The solution used to clean pH electrode depends on the presence of possible contaminants. Mechanically intact electrodes may show slow response due to coating or clogging. Use the guide below to choose the appropriate cleaning solution options:
- For general cleaning: Soak the pH electrode in 0.1 M HCl or 0.1 M HNO3 for 20 minutes. Rinse well in tap water before use.
- For removing stubborn deposits and bacteria: Soak the pH electrode in a 1:10 dilution of household laundry bleach for 10 minutes. Rinse thoroughly before use.
- For removal of oil and grease: Rinse the pH electrode in mild detergent or methyl alcohol. Wash in water before use.
- For removal of protein deposits: Soak the pH electrode in 1% pepsin in 0.1 m HCl for 5 minutes. Rinse well before use.
- For sulfide deposits: Use a solution of 8% thiocarbamide in 1 mol/L HCl.
After any of the cleaning procedures, it is good practice to thoroughly rinse the pH electrode with deionized water.
